Olaparib:Mechanism,Activity,Interactions,Application and Toxicity Studies
Feb 28,2023
General Description
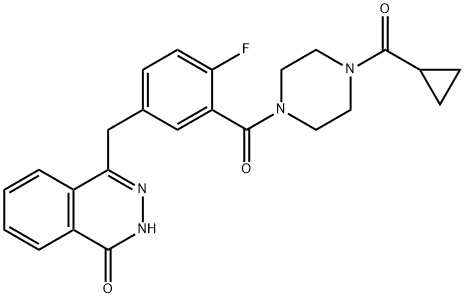
Olaparib represents a novel class of drugs called PARP (Poly ADP-ribose polymerase) inhibitors that primarily interfere with the BER pathway leading to unrepaired SSBs.Olaparib (formerly referred to as AZD2281 or KU0059436) is designated chemically as 4-[[3-[4-(cyclopropanecarbonyl)piperazine-1-carbonyl]-4-fluorophenyl] methyl]-2H-phthalazin-1-one. The molecular formula of this orally active small molecule is C24H23FN4O3 and its relative molecular weight is 435.08g/mol. Olaparib acts mainly as a selective and potent inhibitor of the enzymatic activity of the poly(ADP-ribose) polymerases PARP1 and PARP2 with an IC50 of 5 and 1 nM, respectively. It is rationally designed to act as a competitive inhibitor of NAD+ at the catalytic site of PARP1 and PARP2, both members of the PARP family of enzymes that are central to the repair of DNA single-strand breaks (SSBs) mediated via the base excision repair (BER) pathway.[1]

Figure 1 olaparib capsule
Mechanism
Inhibition of the BER pathway by olaparib leads to the accumulation of unrepaired SSBs, which leads to the formation of deleterious double-strand breaks (DSBs). In cells with an intact homologous recombination (HR) pathway, these DSBs can be repaired effectively.Olaparib binds to the catalytic domain of PARP1 and PARP2, effectively inhibiting PARylation at low nanomolar concentrations. It also exerts anticancer activity by its ability to trap inactive PARP enzymes on DNA, forming toxic PARP-DNA complexes that cause increased double-strand breaks (DSBs).However, in cells with defective HR, the DSBs that result from PARP inhibitor-mediated loss of BER are either repaired by more error-prone DNA repair mechanisms like nonhomologous end joining (NHEJ), single-strand annealing (SSA) and microhomology-mediated end joining (MMEJ), or remain unrepaired, leading to further genomic instability and apoptotic cell death.This phenomenon is referred to as synthetic lethality, where two defects, which alone are benign, can be lethal when combined (e.g., inhibition of PARP activity and loss of DSB repair by HR).[1]
Activity
Olaparib inhibits selectively PARP1 as well as PARP2 activity.It has been demonstrated, that cells containing mutations in BRCA1 or BRCA2 as well as other genes involved in the HR pathway are extremely dependent upon the activity of PARP1 which plays a central role in base excision repair.When PARP1 is inhibited by olaparib, single-strand breaks degrade into double-strand breaks that cannot be repaired due to the defect in HR. Therefore, inhibition of PARP1 confers selective cytotoxicity to tumor cells with attenuated HR function, and several in vitro and in vivo data have demonstrated that cell populations with a known defect in homologous recombination (HR) repair are in fact selectively sensitive to single-agent olaparib.Olaparib has also been investigated in combination regimens, as inhibition of PARP can potentiate the effects of numerous DNA-damaging agents. In a genetically engineered mouse model of BRCA1-deficient mammary tumors, treatment with olaparib and a platinum drug (cisplatin or carboplatin) created potent synergy inducing an increase in overall survival versus either agent alone.[1]
Interactions
Olaparib is primarily metabolized in the liver by the isozymes CYP3A4/5, which can result in drug interactions with CYP3A inhibitors (e.g., macrolide antibiotics, azole antifungals), resulting in increased plasma levels of olaparib. Dose reductions to 150 mg twice daily are recommended for concomitant use of a strong CYP3A inhibitor and to 200 mg for concomitant use of a moderate CYP3A inhibitor.[1]Concomitant use of a strong or moderate CYP3A inducer (e.g., carbamazepine, phenobarbital, rifampicin) should be avoided. If a CYP3A inducer must be co-administered, there is a potential for reduced efficacy of olaparib.Olaparib itself may inhibit CYP3A4 in vitro and it cannot be excluded that olaparib may increase the exposures to substrates of this enzyme in vivo. Therefore, caution should be exercised when substrates of CYP3A4 are combined with olaparib, in particular, those with a narrow therapeutic margin (e.g., simvastatin, cisapride, cyclosporine, tacrolimus).[1]
Application
Olaparib was the first PARP inhibitor to be approved in advanced ovarian cancer therapy for those with germline BRCA1/2 mutations.Olaparib has also shown promising activity in patients with metastatic breast or prostate cancer and a germline BRCA mutation. Besides its usage as a single agent, olaparib can also act either as a chemo-and/or radiosensitizer, due to its ability to potentiate the cytotoxic effects of these therapeutic agents.In 2014, olaparib received approval by the EMA as monotherapy in the maintenance treatment of patients with platinum-sensitive, relapsed BRCA-mutated high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer. Olaparib results in a high antitumor response and disease control rate in BRCA mutation carriers with advanced ovarian cancer.[1]
Toxicity
Dose-limiting toxicities and central nervous system side effects are common dose-limiting toxicity for olaparib, often requiring significant dose modifications of either the PARP inhibitor or the cytotoxic drug.Olaparib has only minimal systemic toxicity as single agents, therefore, representing ideal candidates to act as radiosensitizers.[1,2]At the maximum tolerated dose of 400 mg twice daily, olaparib is comparably well tolerable.The incidence of severe hematologic toxicities varied widely across clinical trials.[3]Severe neutropenia was more common with olaparib plus cytotoxic chemotherapy than with chemotherapy alone, suggesting that the combination therapy might intensify chemotherapy-induced toxicities.[4]A rare, but serious complication in patients who received olaparib represents treatment-related myelodysplastic syndrome/acute myeloid leukemia (MDS/AML).[5]However, the fatigue, nausea and vomiting are the most common adverse events.[1]
References
[1]Bochum S, Berger S, Martens U M. Olaparib[J]. Small Molecules in Oncology.2018: 217-233.
[2]Jackson SP, Bartek J.The DNA-damage response in human biology and disease. [J].Nature.2009,461:1071-1078.
[3]Oza,et al.Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial[J].Lancet Oncol.2015,16 (1):87–97.
[4]Bang,et al.Randomized, double-blind phase II trial with prospective classification by atm protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer[J]. Clin Oncol. 2015 33:3858–3865.
[5]Ricks TK, Chiu HJ, Ison G et al.Successes and challenges of PARP inhibitors in cancer therapy [J].Front Oncol. 2015,5:222.
- Related articles
- Related Qustion
- Olaparib Impurity Standards Jul 7, 2023
Olaparib impurities that include Olaparib Acid Impurity, Olaparib N-Boc Impurity, Olaparib Amine impurity, Olaparib Pyrolidione Adduct, Olaparib Desfluoro Impurity, Olaparib Dimethylbenzamide Impurity, and Diketo Impurity of Olaparib.
Dimethocaine is marketed as local anesthetic in the 1930s and used in dentistry and ophthalmology. It is supplied in the form of a hydrochloride, a white cryst. powder sol. in water up to 50%.....
Feb 27,2023APIDi-Fluoro ethylene carbonate can improve the cycle performance, high temperature performance and flame retardant performance of the electrolyte, is a lithium battery electrolyte additive.....
Feb 28,2023APIOlaparib
763113-22-0You may like
- Olaparib
-

- $1.00 / 1g
- 2024-05-29
- CAS:763113-22-0
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 100kg
- Olaparib
-

- $50.00/ kg
- 2024-05-27
- CAS:763113-22-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 1
- Olaparib
-

- $0.00 / 25Kg/Bag
- 2024-05-27
- CAS:763113-22-0
- Min. Order: 2Kg/Bag
- Purity: 99% up, High Density
- Supply Ability: 20 tons